Today, nuclear physics is one of the most dynamically developing areas of physics. In addition, almost every inhabitant of our planet heard about the terrible tragedy at the Chernobyl nuclear power plant in 1986. This event showed the whole world how dangerous the so-called “peaceful atom” can be. Thus, in this section we will talk about a very interesting, but often dangerous section of physical science.
About 2500 years ago, the ancient Greek philosopher Democritus introduced the assumption that all substances consist of so-called atoms, which in translation meant "indivisible."
But already from the middle of the 19th century, as a result of a multitude of experiments, data began to appear that put this assumption under consideration and indicated the presence of a complex atomic structure.
The impetus to the active research in the field of the atomic structure was the discovery of X-rays, after which many scientists became interested in this topic. After conducting a large number of experiments, some scientists came to the conclusion that X-rays may occur during the short-term luminescence of certain substances after being illuminated by sunlight. Such substances include, for example, certain salts of uranium. One of them was used by the French physicist Becquerel to verify the described phenomenon.
In 1896, he took grains of uranium salt, put them on a photographic plate wrapped in black paper and carried it out for several hours to the sun (photographic plate is a photosensitive photographic material, which is a solid substrate (usually glass) coated with a photosensitive emulsion, Fig. 1 ).

Fig. 1. Photo plate
After developing, dark spots appeared on it, which proved the presence of radiation by uranium salt, which passes through black paper. Then the scientist decided to slightly change the conditions of the experiment, but the weather prevented him, and he put the photographic plate and uranium salt on the table, and between them laid a copper cross. When the weather did not improve after a few days, Becquerel decided to develop a plate and found that the outline of the cross appeared on it. This proved that sunlight has no relation to the effect, and uranium salt itself, without the influence of external factors, emits invisible radiation.
Later, such radiation will be called radioactive (from the Latin. Radio - radiate and activus - effective), the ability of some substances to radioactive radiation - radioactivity, and chemicals whose nuclei are prone to such radiation - radioactive elements.
Later, in 1898, French scientists, Nobel Prize laureates Pierre Curie and Maria Skladovskaya-Curie discovered two new radioactive elements, radium and polonium, whose radiation was much stronger.
After the discovery of radioactive elements, the study of the physical nature of their radiation began. In 1899, under the leadership of the English physicist Ernest Rutherford, it was discovered that radioactive radiation is heterogeneous, that is, as a result, various particles are emitted.
In the picture you can see the diagram of this experiment.
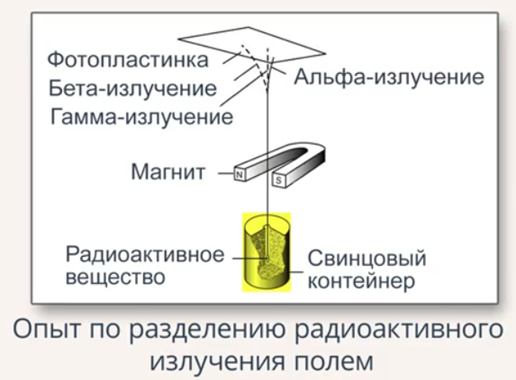
Fig. 2. Experience
A radioactive substance was placed in a lead container with a small opening. Lead allowed to shield the radiation in all directions, except the hole. The radiation beam that came out of the hole first fell into the strong magnetic field of the permanent magnet, and then onto a photographic plate placed opposite the hole. On the photographic plate after the manifestation, three dark spots were detected, which testified to the division of radioactive radiation into three components.

Fig. 3. Illustration of experience
First of all, two spots, deflected in different directions from the rectilinear propagation of the beam, indicated the presence of opposite charges on the particles in these beams. The third spot was located opposite the hole, it was concluded from this that the radiation of this beam had no charge.
Charged particles in bundles Rutherford was able to explore. Positively charged were the nuclei of helium atoms, he called them α-particles, negatively charged turned out to be fast-moving electrons, which he called β-particles.
In 1900, the French physicist Paul Willard discovered that component of radiation, which had no charge, it was called γ-radiation. The study of these rays showed that they are electromagnetic waves of extremely high frequency and energy.
So, let us once again list all of the particles that are released during the process of radioactive radiation:
- α-particles - helium nucleus flux  ;
;
- β-particles - a stream of fast electrons  at a speed comparable to the speed of light;
at a speed comparable to the speed of light;
- γ-radiation - high-frequency electromagnetic radiation.
As a result, the phenomenon of radioactivity was the basis for assuming that atoms have a complex composition.
In today's lesson, we learned about the history of the discovery of radioactivity, as well as about the experiments of Rutherford, through which three types of radioactive radiation were discovered.
In the next lesson we will look at models of atoms and other experiments of Rutherford.
Bibliography
- Bronstein MP Atoms and electrons. "Library" Quantum "". Issue 1. - M .: Science, 1980.
- Kikoin I.K., Kikoin A.K. Physics: Textbook for grade 9 high school. - M .: "Enlightenment."
- Kitaygorodsky A.I. Physics for all. Photons and nuclei. Book 4. - M .: Science.
- Rutherford E. Selected Scientific Works. Radioactivity. - M .: Science.
- Rutherford E. Selected Scientific Works. The structure of the atom and the artificial transformation of elements. - M .: Science.
- Filatov E.N. Physics 9. Part 1. Kinematics. - The Avangard Higher School of Foreign Affairs.
Additional recommended links to Internet resources
- Youtube.com ( A source ).
Homework
- Who first observed the radioactive radiation of uranium?
- How were the new chemical elements capable of spontaneous radiation discovered by the Curie spouses called?
- What is radioactivity?
- Who first introduced the term "radioactivity"?
What is radioactivity?
Who first introduced the term "radioactivity"?
 МОЯ ТВОРЧЕСКАЯ ЛАБОРАТОРИЯ
МОЯ ТВОРЧЕСКАЯ ЛАБОРАТОРИЯ